Security Requirements and Controls
Security requirements define what must happen. Controls define how. This distinction is critical for safe, secure product development.
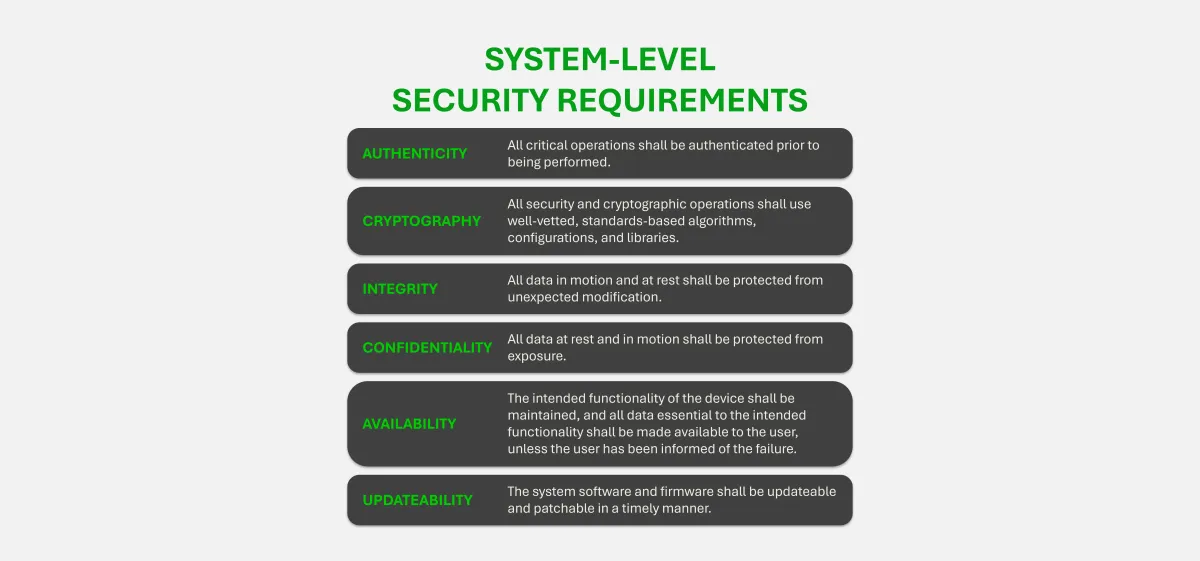
Security requirements define what must happen. Controls define how. This distinction is critical for safe, secure product development.
AcmeStim Systems A Cybersecurity Case Study of FDA Compliance with Velentium Medical

The 12 Required Cybersecurity Artifacts for an FDA Submission

Demystifying FDA Cybersecurity Requirements for Medical Devices
Safe. Secure. Effective.
One stop for secure Medical Device R&D, product development, contract
manufacturing, and postmarket services
Who We Are
© 2026 Velentium Medical LLC. All Rights Reserved.